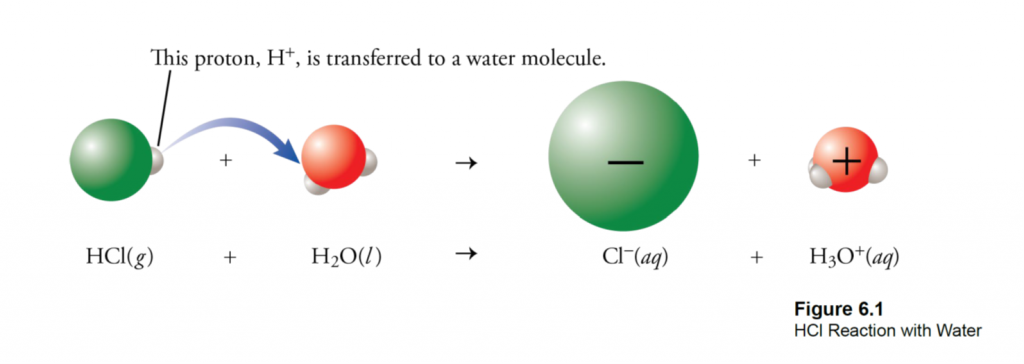
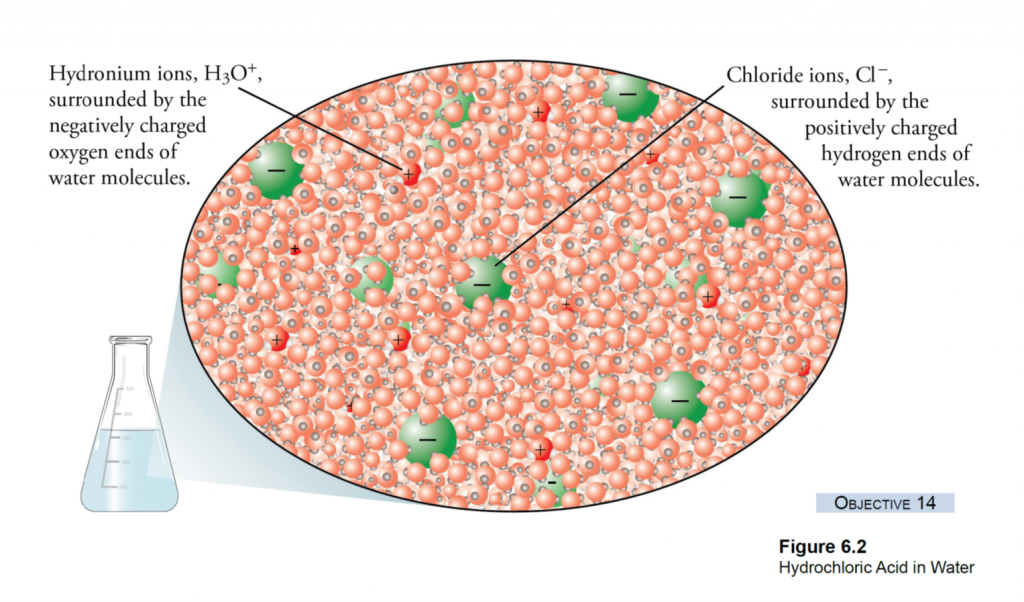
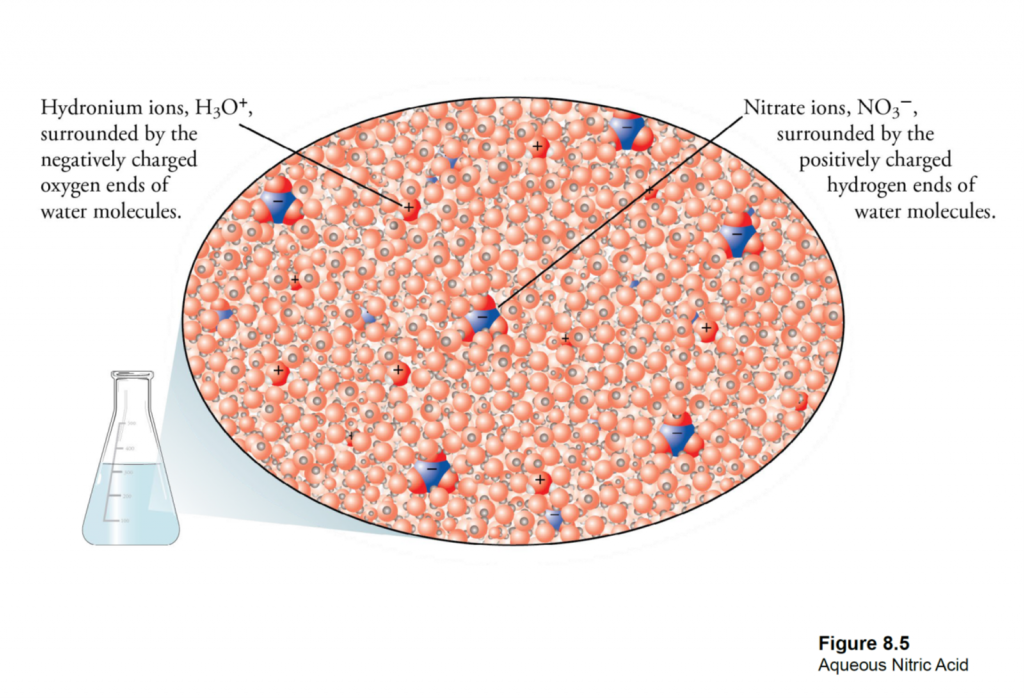
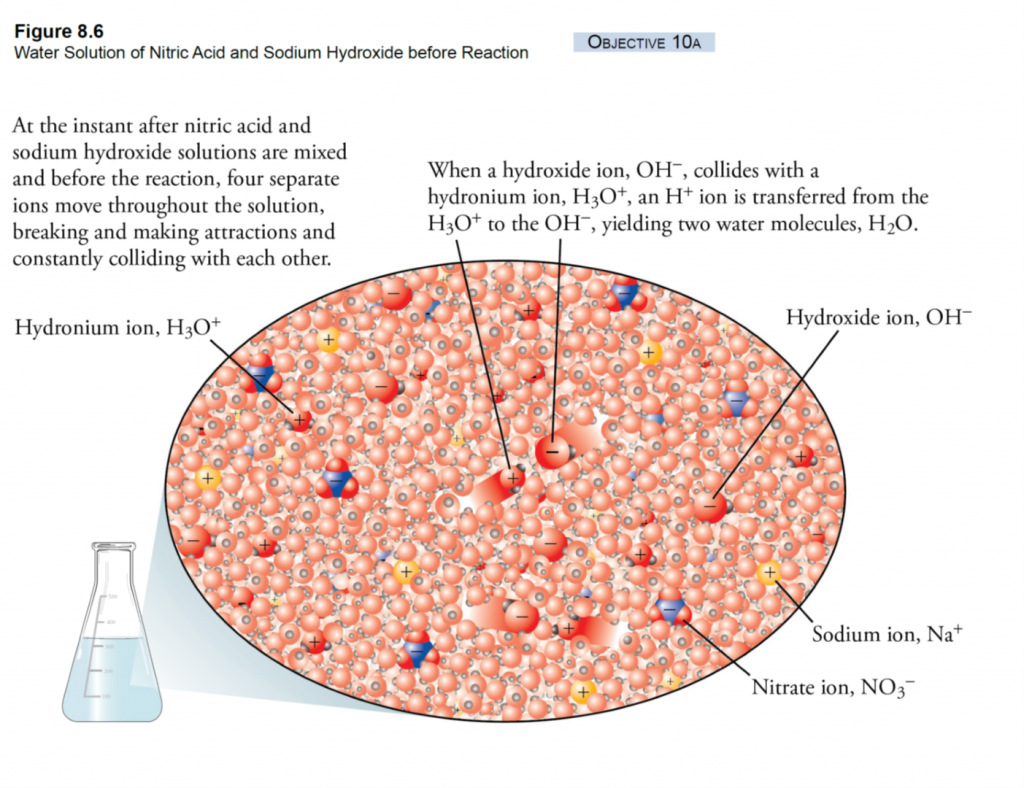
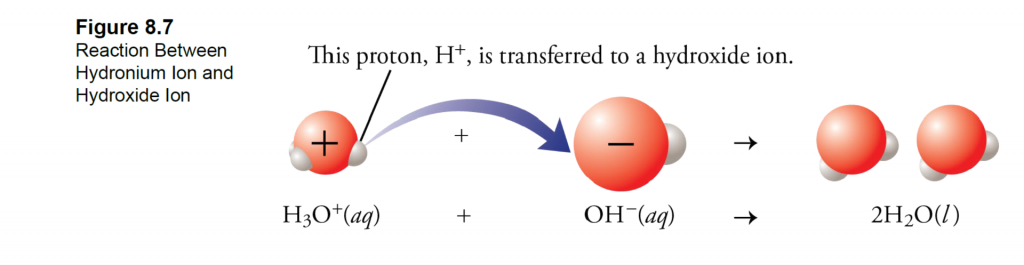
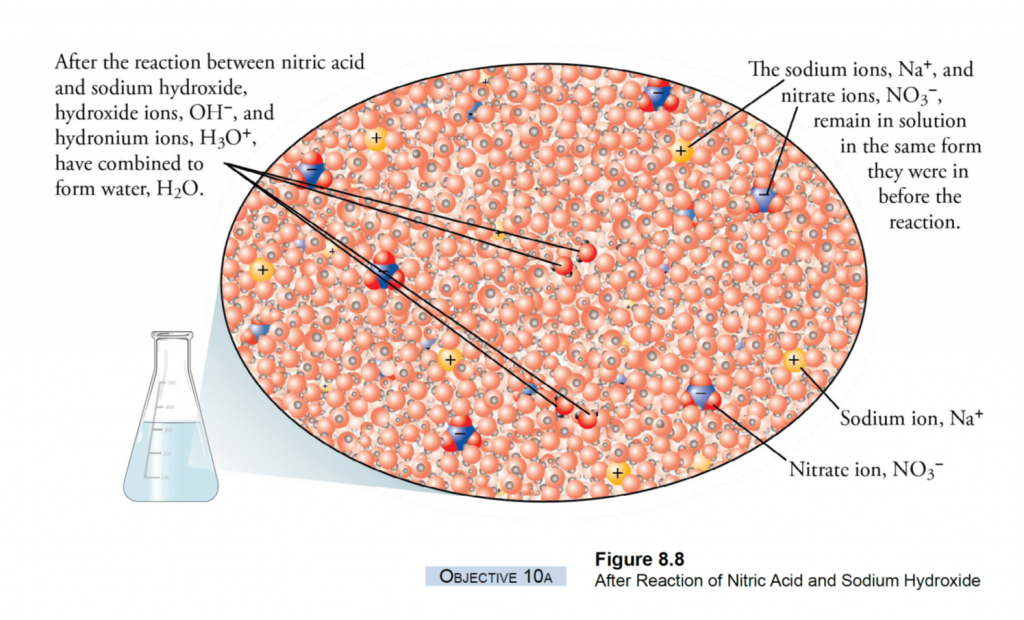
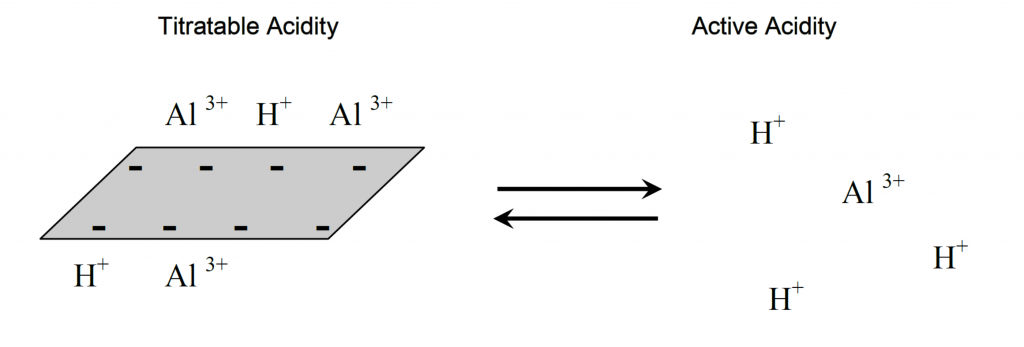
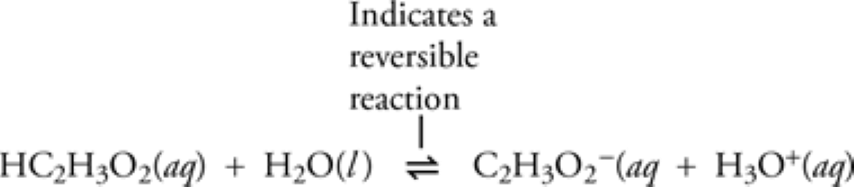What is Calcareous Clay?
posted by Karen Orlandi on July 1, 2016 in Wine IQ
Many vineyard sites, especially the top tier french chateaux and domaines, describe their soil type as calcareous clay. Calcareous is actually an adjective meaning “mostly or partly composed of calcium carbonate.” Other synonyms for calcium carbonate include lime or chalk. Due to the low acidity level of calcium carbonate, the calcareous clay soil is quite alkaline compared to other soil types. The biggest benefits of calcareous soil are the special nutrients it supplies to the grapes, which make them grow better and sweeter. It also has the effect of keeping the soil at a cooler temperature, which can be helpful on hot days, but it also usually delays ripening, yielding a more acidic wine. The clay portion of the soil retains moisture, which helps the grapes during dry weather.

| Oamaru Shallow Clay Loam | Southland | Typic Rendzic Melanic Soil | Lithic Rendoll | 0 – 5 cm: Black calcareous clay loam, fine spheroidal structure, friable, non-sticky. |

| Bluff Clay | Hawkes Bay | Calcareous Orthic Melanic Soil | Typic Rendoll | 0 – 30 cm : Very dark brown clay, strongly pedal, fine polyhedral macrofabric. |

Excerpts- papers on nutrition in calcareous soils
Food Availability in Alkaline-Calcareous Soils (pdf)
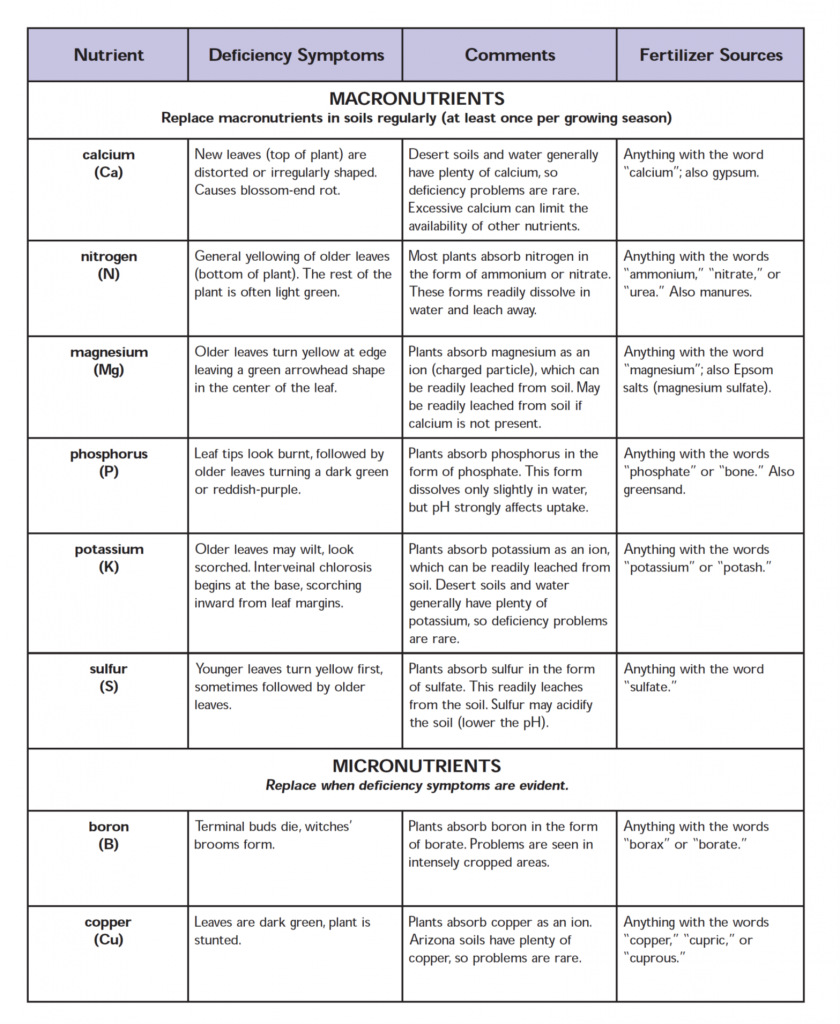
The alkaline-calcareous soils of Arizona contain large reserves of phosphate, potassium, and calcium. The availability rating of the phosphate reserve is very low under alkaline and calcareous conditions. The potassium reserve has a high availability rating. A large reserve of calcium is present as in the form of caliche, with appreciable amounts present as replaceable calcium, and as soluble calcium salts in the soil solution.
SUMMARY- TECHNICAL BULLETIN NO. 94
Before the question of efficient use of fertilizers can be ap- proached intelligently it is necessary to have regard- ing the availability of fertilizer materials, their fixation in soils, and the influence of the many growth limiting or growth promot- ing factors which affect crop growth on irrigated lands. This investigation has added considerably to our knowledge of the solubility of the phosphate, potassium, and calcium compounds in alkaline-calcareous their physiological availability, and some of the factors influencing this, and the comparative quantitative value of the Neubauer and carbonic acid methods for estimating this availability. Due to the difference in chemical composition of the phosphate, and calcium compounds and minerals, as well as the fixation of these elements, their avail- ability is affected by a complex set of factors. The most tant single physiological affecting all is the buffer capacity of the for this materially influences the H-ion concentration in the root-soil contact film. It is within this boundary that plant food elements are converted from insoluble forms to physiological availability. This condition is magnified under alkaline-calcareous soil conditions, for all these soils are highly buffered.
has been frequently mentioned phosphate availability is the major plant food availability problem in alkaline-calcareous soils. In most part, the reserve phosphate in these soils is present as calcium carbonate-phosphate,
The availability of this compound, which is naturally very low, is further reduced by solid phase (caliche) and sodium and calcium salts which are present in the soil solution, factors which are abundantly present in southwestern soils. All these factors reduce both the solubility and the absorption of phosphate ions by plant roots. A small part of the soil phosphate is probably present in replaceable as shown by its solubility in alkali and the abundant soluble phosphate which exists in black alkali soils where hydroxyl ions exist in excess in the soil solution. On the other hand, since plant roots cannot assimilate phosphate ions in the presence of an excess of OH ions, this soluble phosphate in black alkali soils is of little use to crops unless [the pH] of the soil is lowered.
In view of the above, soluble phosphate fertilizers, at least nothing less soluble than single superphosphate, are recommended for these calcareous soils. Even the soluble phosphates are im- mediately fixed largely as tricalcium phosphate. According to Naftel (15) monobasic calcium phosphate, exists only within the pH range of 3.0 to 5.0; dibasic calcium phosphate, at pH 5,0 to 6.4; and tribasic, above pH 6.4.
It seems reasonable to suppose then that most of the soluble phos- phate used on Arizona soils is rapidly fixed as tribasic calcium phosphate. Since this fixed phosphate has been shown to possess contact availability and to maintain this state of availability over an extended period, it is apparent that the final conversion to calcium carbonate-phosphate is a very slow process. The evidence indicates then that soluble phosphates are fixed as tribasic cal- cium phosphate and that the reserve naturally in the soil is largely calcium carbonate-phosphate.is present in the soil minerals largely in nonreplaceable and insoluble forms. Replaceable potassium is however present in sufficient abundance to meet present needs, andthe potassium problem is, as yet, not a serious one on Arizona soils.
Calcium is present in greatest part as with lesser amounts as replaceable calcium and soluble calcium salts in the soil solution. It has been generally assumed that calcium nutrition is not a problem of any consequence in calcareous soils. However, due to the great difference in solubility of and soluble calcium is very low in the more alkaline soils where bicarbonates do not exist. The investigations in this laboratory have shown a wide variation in the amount of calcium taken up by plants from calcareous defin- itely proving a variant physiological availability. In many of these calcareous notably the most alkaline negative absorption of calcium by plants was observed for the seedlings grown in the Neubauer test.
For estimating the availability of these three elements a study involving a comparison of numerous plant cultures and the car- bonic acid method, for chemical soil analysis, has been made. While the absorption of phosphate, potassium, and calcium from soils is different for plants, where a large number of soils are compared, and the data graphically presented, the rel- ative absorption is in good agreement. The Neubauer method, using the rye seedlings, can therefore be used as a measure of availability for many crops. Criticism of the method, on this basis, does not appear to hold valid for alkaline-calcareous Experiments do, however, show that if the carbonic acid method is, like other chemical methods, to be classed as empirical, then the same empiricism must be true for the Neubauer method. The carbonic acid method and the Neubauer method agree quite well in availability tests on calcareous soils.
It is shown that the Neubauer plant test is not strictly a method for determining the amount of available plant food in the soil, in all plant tests on irrigated soils other growth limiting will affect the results. It may be better referred to as a measure of physiological availability. For interpreting the value in terms of other crops some consideration must be given to their tolerance the growth limiting which commonly exist in irrigated soils.
The carbonic acid method is specifically a measure of solubility or availability without consideration the which deter- mine physiological availability or absorption. With a knowledge of the tolerance of crops for these factors the carbonic acid method has considerable intrinsic merit for alkaline-calcareous soils and appears to be at least on a par with the Neubauer test.
The empirical nature of the Neubauer test is shown by the re- sults obtained from the experiments where the number of plants was kept constant at 100 and the weight of soil in each culture was On the whole most of the soils showed closely constant
values above 100 plants per 80 grams of soil. One hundred plants per 100 grams of soil appears therefore to be a fair arbitrary proportion.
There are certain advantages in both the Neubauer and carbonic acid methods measuring available plant in soils. With the Neubauer method affecting physiological absorption come into while the carbonic acid method makes no measure of these measures only the available plant food and consideration must be given to growth limiting factors. A
SEEDLING TESTS AND SOIL ANALYSIS 415
study of some of these factors showed that pH of the soil and its related influence has a major effect on absorption of phosphate, and calcium by the plants. All increased as the pH of
soil decreased. Of the soluble salts present in irrigated soils the calcium salts have the greatest influence on and this is even true for calcium carbonate which is only very slightly soluble.
The empirical nature of the Neubauer method was also shown by experiments in which the weight of soil and number of plants per culture were varied. The magnitude of the value, on a 100 plant decreases with increase in weight of soil and number of rye plants used in the cultures. In other words the extraction of phosphate, potassium, and calcium per plant, from the soil, is greater the smaller the number of plants grown. For alkaline- calcareous soils the ratio of 100 plants to 100 grams of soil may be accepted as useful, although not the best ratio. Using weights of soil varying from 10 grams to 200 grams per 100 seedlings the extraction reaches a maximum at 80 to 100 grams soil. The phosphate extraction remains practically constant above this. No sat- isfactory explanation of this constancy can be offered at this time, although several suggestions have been presented in the text. The minimum Neubauer value for soils is 10.6 yet few Arizona soils except those with phosphate reach this minimum. This is true regardless of the fact that in these experiments as high as 200 grams of soil per 100 plants were used, and still the values not only failed to reach the value of 10.6 but even showed no increase above the value obtained for 100 grams of soil. This indicates that smaller weights of soil might be more desirable for the Neubauer test on alkaline-calcareous possibly 50 grams soil per 100 plants. This ratio of weight of soil to number of plants would be on a point well below the 100/100 ratio and within the range where the curve is increasing with in- crease in weight of soil rather than at the point where the value has become constant.
The above condition does not hold true in entirety potassium. The magnitude of the Neubauer values, like the values, does decrease with increase in number of plants and weight of soil per culture. Also on maintaining the number of plants constant at 100 and increasing the weight of soil from 10 grams to 200 grams the K values become practically constant at 100 grams of soil. However, the values do not become constant until they have extended well beyond the minimum K value of namely, 19.9 mgm. K. Potassium availability then in alkaline- calcareous soils is from that of phosphate where regard- less of weight of soil above a certain soil-plant ratio few soils will reach the minimum value established by Neubauer himself.
The addition of soluble phosphate fertilizer to the cultures showed that phosphate fertilization will increase the absorption f potassium by rye plants and decrease the absorption of calcium, depending somewhat upon the amount of phosphate added.
On the basis of previous phosphate investigations only soluble phosphate fertilizers are recommended for Arizona soils. Even these phosphates are immediately fixed and do not move far from the place of application. For this reason a part of investiga- tion involved a study of the availability of this fixed phosphate. It appears to be a precipitation in the form of tribasic calcium phosphate with some sorption by the soil colloids. The data show definitely that while the fixed phosphate is not mobile in the soil solution it is readily available when in contact with feeding roots. Both the Neubauer method and the carbonic acid extraction method are in measuring this fixed phosphate in calcareous soils.
CONCLUSIONS
1. A comparison of the quantitative removal of phosphate, and calcium from soils by rye, wheat, cowpeas, tomatoes, and corn show a good
relative agreement for rye, barley, wheat, and tomatoes and a fair relative agreement of rye with corn, and hegari.
2. There is a good correlation between the availability of phos- phate, calcium, and potassium as measured by the carbonic acid method and the availability values obtained from the Neubauer test.
3. Some factors which are related to or which influence the ab- sorption of these three ions are as
a. Phosphate absorption increases with decrease in pH, decrease in water soluble calcium, carbonic acid soluble calcium, and calcium carbonate.
b. Potassium absorption increases with decrease in pH value.
c. Calcium absorption increases with decrease in pH value.
d. Phosphate Neubauer values correlate with the carbonic acid soluble phosphate and with the Ca Neubauer values.
e. Potassium Neubauer values correlate with increase in Ca Neubauer values, carbonic acid soluble potassium, and replace- able potassium.
f. Calcium Neubauer values correlate with carbonic acid sol- uble calcium, and calcium carbonate but inversely with replace- able calcium.
4. In varying the weight of soil in each 100 plant Neubauer culture there is a steady increase in value or per 100 plants up to 80 to 100 grams of soil, above which the value is closely constant.
5. For potassium the Neubauer K value is closely constant above 100 grams of soil.
6. For calcium the Neubauer value curve shows less constancy, reaching maximum values at 40 to 90 grams soil, beyond which it decreases.
SEEDLING TESTS AND SOIL ANALYSIS 417
7. When both number of plants and weight of soil are varied in the Neubauer test, the value on the basis of 100 plants de- creases as the number of plants and weight of soil are increased.
8. When weight of soil is held constant and number of plants increased, there is a decrease in Neubauer value on a basis with increase in weight of soil.
9. The suggestion is offered on the basis of this investigation that even though the results obtained with a 100 plant 100 grams of soil ratio are useful and arbitrary, using lesser amount of soil might enhance its value. The curves obtained, both phosphate and potassium, on plotting values obtained by maintaining the number of plants constant at 100 and increasing the weight of soil per culture, show practically horizontal curves above 80 to 100 grams of soil. In other words the absorption is at a maximum and practically constant above this. It is believed that some point below 100 where the values are steadily increasing, possibly 50 grams of soil, would give more satisfactory values.
Phosphorus Availability with Alkaline/Calcareous Soil (pdf)
Phosphorus (P) is an essential nutrient required by plants for normal growth and development. The availability of P to plants for uptake and utilization is impaired in alkaline and calcareous soil due to the formation of poorly soluble calcium phosphate minerals. Adding fertilizer P at “normal” rates and with conventional methods may not result in optimal yield and crop quality in these soils common in arid and semi-arid regions. Several fertilizer P management strategies have been found to improve P nutrition for plants grown in alkaline and calcareous soil. Research results show that relatively high P fertilizer rates are required for crops grown in alkaline soil, with increasing rates needed as lime content in these soils increases. Concentrated P fertilizer bands improve P solubility with resulting yield increases, even when applied to crops grown in soil with relatively high soil test P concentrations. Applying organically complexed P in the form of biosolids or as a mixture of liquid P and humic substances can also enhance P nutrition and result in yield increases. Application of slow release and cation complexing specialty fertilizer P materials has also been shown to effectively increase yields in calcareous soil. In-season applied P through the irrigation water can deliver P to plant roots when deficiencies are observed, but the effectiveness and results are less than with P incorporated into the soil. Finally, it is important to maintain a proper balance of P with other nutrients for general plant health and to avoid excess nutrient induced deficiencies of other nutrients. In some cases, these methods are relatively new and need further refinement with regard to rates, timing, and technique; but all are potential methods for improving P supply to plants grown in alkaline and calcareous soil.
IN-SEASON P FERTILIZER APPLICATION
Substantial research on nitrogen in potato production shows the value of split applying this nutrient, with a majority applied during the peak uptake rates during the season (Stark and Westermann, 2003). Nitrogen fertilizer quickly converts to the nitrate form, which is easily leached or volatilized under conditions with ample soil water. Furthermore, nitrate does not form poorly soluble minerals and, thus, is not subject to solubility problems. Alternatively, P is not typically subject to leaching loss and does not volatilize. And, as mentioned previously, P readily forms poorly soluble soil minerals. Therefore, the chemistry of P does not lend itself to movement in the soil and, as such, it is reasonable to assume that P should be incorporated into the soil zone where roots grow and take up nutrients.
At times, tissue analysis shows that a crop is potentially P deficient despite pre-season fertilization efforts. Can P be applied in-season to some benefit? Recent research in potatoes shows that, although not as efficient as pre-plant broadcast/incorporated fertilizer, P can be applied with some benefit during the season as an injection in the irrigation water (Hopkins and Ellsworth, 2003). However, it is recommended that every effort should be made to supply adequate P with other methods and use the in-season P application as a last resort if tissue analysis shows deficiency. Foliar applications of P may also be beneficial, but should not take the place of a good pre-plant fertilizer P program (data not shown).
BALANCING P WITH OTHER NUTRIENTS
Adding P in combination with ammonium tends to enhance availability of both nutrients. Ammonium and other acidifying fertilizer materials can enhance P solubility and uptake by roots. In general, it is also wise to have generally sufficient nutrient levels of other nutrients to promote overall good root development, which is so important for P interception and uptake.
Excessively high levels of certain nutrients can induce deficiencies of others. High rates of zinc, iron, manganese, and copper can induce deficiencies of P and visa-versa. This effect has been observed in many crops. Recent work in Idaho shows that excess P can result in potato yield and quality loss, which may be overcome with addition of zinc fertilizer (Hopkins and Ellsworth, 2003). Although this effect was observed by other researchers and in one year of this trial, no yield loss was observed at rates as high as 600 lbs P2O5/acre in two other years of the trial. There are also many claims with regard to optimum P-micronutrient ratios, but these purported ratios are not generally based on experimental data collected under field conditions. In fact, it is common to see a variety of soil P-micronutrient ratios in fields with exceptionally high yields, leaving the concept of optimal ratios in doubt. More work is needed to determine if optimum ratios exist and, if so, the width of this range.
SUMMARY
Phosphorus is an important and essential nutrient for all plants. Availability of P in high pH soils, especially those with excess lime, is relatively poor. Lowering pH is not an economical option for most crops and, as such, other strategies must be employed to enhance P uptake by roots, including: 1) relatively high P fertilizer rates, 2) concentrated P fertilizer bands, 3) complexed P fertilizer, 4) slow release fertilizer P, 5) cation complexing P fertilizer, 6) in-season P fertilizer application, and 7) balancing P with other nutrients.
Plant-soil-nutrient status of vegetables and wheat grown oncalcareous soil (pdf)
Calcareous soil is extremely important in determining nutrient status and availability when applying different fertilizers to different crops associated with or without irrigation. The objective of this study was to investigate the nutritional status (nutrient availability) of different plants grown in two different ecosystems dominated with calcareous soil : Ghor Alsafi (eggplants, tomato and beans) as an example of irrigated area and the Karak Mountain Area (wheat) as an example for non-irrigated area. Physiochemical properties in soils and nutrients concentrations (C, Fe, Mn, Zn, available P, available and non-available K) in soil and plant leaves were analyzed. This study showed that N concentration in soil from both Ghor Alsafi (ranged from 4.4-5.1 mg/g) and the Karak Mountain Area (1.9-3.2 mg/g) was relatively low. This study showed that Fe content in vegetables grown in Ghor Alsafi was about 3 to 5 fold higher than the recommended maximum Fe concentration. The concentration of other nutrients (N, K, Zn and Mn) in investigated vegetables fell within the recommended concentrations range (38.3-39.7 mg/g, 36.6-39.5 mg/g, 21.7-27.3 ppm and 49.2-102.6 ppm, respectively). Phosphorus content in vegetables grown in Ghor Alsafi and in wheat grown in different locations in the Karak Mountain Area was about 5?6 fold higher than the recommended P concentration. Our results indicated that the high levels of P (ranged from 3.28-3.43 mg/g) and Fe (ranged from 340.4-551.3 ppm) in vegetables grown in Ghor Alsafi and high levels of P (ranged from 2.2-2.52 mg/g) in wheat in the Karak Mountain Area can be attributed to high levels of fertilizer application.
CONCLUSION
It can be concluded from this study that farmers in both the areas studied (Ghor Alsafi and the Karak Mountain Area) used improper cultivation practices, which was more noticeable in Ghor Alsafi. The continual addition of P rich fertilizer increased the P content in soil above the optimum level required by crops. This study revealed that farmers in the Karak Mountain Area did not use enough K rich fertilizers. Crop rotation could be an effective approach to maintain soil fertility in the mountain area. Further research should be conducted to minimize nitrogen losses through the volatilization of NH3 from calcareous soil
Nitrogen Management in Calcareous Soils: Problems and Solutions (pdf)
Nitrogen (N) is the most widely applied plant nutrient and is a key constituent of animal manures. Improvement in crop yields and high economic returns have been made possible through supplementation of crops with organic and inorganic fertilizers. The movement of N in the environment has been extensively studied and documented. The fate of N ranges from nitrification, denitrification, nitrous oxide formation, leaching of nitrate, and volatilization of ammonia. Even with this knowledge the variation in N movement within the soil, air and water varies greatly with changed edaphic factors. For instance the fate of N fertilizer under calcareous soil systems still has not been investigated widely. We reviewed the sporadic information available on N fate and management in calcareous soils especially in Pakistan. We discussed the sources and fate of N in calcareous soils of Pakistan and also the currently adopted and newly developed method to reduce the N losses.
Conclusion:
Even though extensive research has been conducted on N dynamics and management worldwide, still a lot of gaps are present in information regarding fate of N fertilizers in soil and its impact on crop growth and environment in Pakistan’s agricultural systems. Most of work found was conducted under controlled conditions in pot and lab trials that lack field validation. According the published data available, nitrogen use efficiency (NUE) for major crops including wheat, maize and rice seldom exceeds 40%. In Pakistan, about 22 to 53% NH3 is predisposed to atmosphere due to high soil pH and hot climatic conditions. Studies have also shown that soil moisture content and salinity have a combined effect on increasing NH3 volatilization. The second major source of N losses in Pakistan’s agro-climatic conditions is de-nitrification. The ammonium fertilizers are quickly nitrified because of warm climate and NO – is vulnerable to denitrification following reduced conditions or flooding.
Nitrogen losses through volatization may be reduced by upto 80% by adopting a few management practices such as application of irrigation after 8 hours of urea application, placing the urea below 3-5 cm below the surface under moist soil conditions and urea co-applied with potash. Use of urease inhibitors such as N-(n-butyl) thiophosphoric triamide (NBPT) or phenyl phosphoro diamidate (PPD) and polymer coating of N fertilizers have shown significant reduction in volatilization. However, information is lacking on their effects under various cropping systems in Pakistan. More recently the use of cheap natural plant products or byproducts including neem (Azadirachta indica), Karanj (Pongomia glabra), vegetable tannins, waste products of tea, mint oil, Japanese mint and Mustard (Brassica juncea L.) are being used as nitrification inhibitors and to increase the NUE (Personnel Communication). However, no extensive research has been conducted on use of these natural substances in the field which is direly needed. Research is still lacking in understanding and quantification of N budgets in different cropping zones and scientist should conduct research on this aspect as well as test new and cheap means to reduce the losses of N in calcareous soils of Pakistan.
Nitrogen Application in Warm, Dry Weather
e have been seeing relative warm, dry, windy weather in many parts of the state over the last week or so, which has raised some questions about higher potential for ammonia-N volatilization loss from surface applied urea containing fertilizers. Applying any urea containing fertilizer to the soil surface during warm, dry, windy conditions will maximize the potential for N volatilization losses. This loss occurs quickly, starting within hours following application with most of the loss occurring within 2 days following application. If the N is going to be incorporated by tillage or rainfall, it is critical that tillage be done or rainfall occurs as soon as possible following application. To minimize losses this should happen within a day following application. One strategy is to delay application a few days if rain is in the forecast. However, an important consideration is, that under generally dry conditions with occasional small showers, a little bit of rain, a few tenths of an inch, can actually make the loss worse because it is just enough to dissolve the urea and activate the volatilization process, but not enough to soak the urea into the soil to minimize the loss. Management options under these conditions include immediate incorporation of the urea by tillage. In no-till or where immediate tillage is not possible, there are other options.
Nitrogen in the Environment: Denitrification
oil microorganisms need oxygen for fuel. When the soil is very wet, water fills in the spaces between soil particles. This leaves very little room for oxygen. Some soil microorganisms can get the oxygen they need from the oxygen portion of the nitrite (NO2-) and nitrate (NO3-) forms of nitrogen. When this happens, nitrogen (N2) and nitrous oxide (N2O) gas are formed. These gases return to the atmosphere, and there is a net cycle in the soil. This is called denitrification
Two main factors influence denitrification:
- The oxygen supply in the soil.
- The soil microorganisms.
Anything that changes these two factors will change how much nitrogen is lost and how fast this happens. These factors include the amount of organic matter, soil water content, soil oxygen supply, soil temperature, soil nitrate levels and soil pH.
A small amount of denitrification can be found taking place in soils all the time. Denitrification becomes significant when the soil is waterlogged for 36 hours or more. The longer the soil is waterlogged, the greater the potential loss of nitrogen from the soil system.
…
Once nitrates get into the groundwater, the greatest concerns are for infants less than one year old and for young or pregnant animals. High levels of nitrates can be toxic to newborns, causing anoxia, or internal suffocation. Seek alternative water sources if nitrate levels exceed the health standard of 10 ppm nitrate-N. Do not boil water to eliminate nitrates. It increases nitrate levels rather than decreases them. The most common symptom of nitrate poisoning in babies is a bluish color to the skin, particularly around the baby’s eyes and mouth. These symptoms of nitrate toxicity are commonly referred to as the “blue-baby” syndrome.
Soil–Plant Nutrient Interactions on Manure-Enriched Calcareous Soils (pdf)
As there are a limited number of ion carrier sites on root plasma membranes, ions with similar diameters and ion strength can outcompete each other for space on these sites (Marschner, 2011). Potassium is known to be a strong competitor against Mg, Ca, and Na, and can restrict uptake of these nutrients when in abundant supply (Mengel, 2007). In addition to P, dairy manures also contain high quantities of K, which is a common dairy feed additive. In relation to fertilizer sources, excessive quantities of K has decreased Ca and Mg tissue concentrations in forage sorghum [Sorghum bicolor (L.) Moench] (Reneau et al., 1983) and in corn (Claassen and Wilcox, 1974), decreased Mg tissue concentrations in sorghum (Ologunde and Sorensen, 1982), and triggered Mg deficiencies in orange trees (Citrus spp.) (McColloch et al., 1957). Parsons et al. (2007) found significant reductions in Ca uptake by wheat receiving manure applications in comparison to fertilizer or an unamended control. The authors noted that Ca uptake may have been hindered by competition with K. Leytem et al. (2011) also found reduced Ca uptake in corn with increasing dairy manure application rates. In addition to cation competition, the authors suggested that the reduction in Ca uptake on manure-treated soils may also be related to the formation of Ca-phosphate precipitates.
Excess K application may also increase the potential for lu xury K consumption by plants. When K supply is abundant, luxury K consumption is known to be an issue in many crop plants (Marschner, 2011). Unfortunately, dairy cows that consume forage tissue with K concentrations of 1.5% or greater are at risk of milk fever (Penn State University Extension, 2013), explaining why many animal producers are concerned with luxury consumption of K by forage crops. As described above, K is known to accumulate in manure-treated soils, and could therefore be a potential issue for growers who use their crop as an animal feed.
conclusion
From this research, we found correlations between soil nutrient levels and tissue nutrient levels for corn silage grown on nutrient-enriched calcareous soils with reported dairy manure application histories.
Specifically, we found positive linear correlations between
soil test K and tissue N,
soil test B and tissue N, and
soil test K and tissue K
concentrations, and found a significant negative inverse relationship between
soil test Fe and tissue Mn concentration.
These findings suggest that growers producing corn silage on alkaline soils receiving dairy manure applications should consider monitoring plant tissue for N, K, and Mn concentrations to avoid reaching toxic (N or K) or deficient (Mn) levels. Our findings also suggest that interactions such as P–Zn and cation competition between K, Ca, and Mg may not be a major issue on nutrient-enriched alkaline and calcareous soils with dairy manure application histories. Finally,
there were significant soil accumulations of [N], K, Fe, Zn, and B associated with increasing Olsen P accumulations, suggesting that dairy manures are significant sources of these five nutrients in the Snake River Region of southern Idaho. Controlled studies are needed to further validate the interactions found in our study.
Legumes: Model Plants for Sustainable Agriculture in Calcareous Soils (pdf)
The most typical root responses of plants in increasing the solubilisation of soil P is the decrease in rhizosphere pH by the release of H+ and phosphatases acids to the rhizosphere [12]. Enhancement of acid phosphatase activity (APases) under P starvation conditions has been demonstrated for N2- fixing legumes including Vicia faba [12], Lupinus albus and Glycine max [13, 14]. Studies in Vicia faba and Vicia sativa showed that P deficient plants increased both extracellular APases, which are involved in hydrolysis of soil’s various organic phosphate monoesters, and intracellular enzymes acting in the remobilization of Pi from rich P components inside the plant cell [12].
With P deficiency, the concentration of phenols increases to improve the availability of the sparingly soluble soil P for plants [15]. In Vicia faba, the exudation of phenolic compounds have been reported a higher concentrations in P-deficient plants [12]. Likewise, it has been demonstrated that Lupines albus and Brassica napus released large amounts of phenolics into the rhizosphere in response to P deficiency [16]. The maintenance of the P-homeostasis in nodules is considered as a main adaptive strategy for rhizobia- legume symbiosis under P starvation by increasing P allocation to nodules, formation of a strong P sink in nodules and direct P acquisition and remobilization [15].
In the face of Fe deficiency widespread in calcareous soils, legumes have evolved adaptive strategies in order to tolerate the stress and increase Fe bioavailability [15]. The main adaptive responses are the rhizosphere acidification by the activation of plasma membrane H+-ATPase, the stimulation of the reduction of Fe3+ to Fe2+ by a NADPH-dependent Fe (III)-chelate reductase (FCR) and the stimulation of root exudates [17]. Several research have been described the genetic variability among legumes regarding their tolerance to Fe starvation. In fact, there are numerous studies aimed to identify and select legume species and genotypes tolerant to Fe deficiency with the potential to increase agriculture production in calcareous soils [18]. assessed the genetic variability among 12 cultivars of peanut (Arachis hypogaea L.) in tolerance to iron deficiency based on spectral and photosynthetic parameters. These authors selected three groups according to their tolerance to Fe deficiency:
conclusion
In arid and semiarid regions, alkalinity is an important environmental constraint for nutrients bioavailability especially Fe and P, which leads to lower crop production. Factors that contribute to Fe and P deficiencies in calcareous soils are high pH values and bicarbonate concentrations. Improving crop production in such soils demand adoption of special management practices which aims to ensure sustainable agriculture that respects the environment. The introduction of legumes in calcareous soils sustain productive agriculture. Hence, it is primordial to select rhizobia strains and legumes genotypes with enhanced tolerance able to thrive under nutrient limiting conditions and subsequently the use of these selected genotypes in crop rotation.
Iron deficiency and chlorosis in orchard and vineyard ecosystems (pdf)
A significant part of the fruit tree industry in Europe and especially in the Mediterranean area is located on calcareous or alkaline soils, which favour the occurrence of Fe chlorosis. Fruit trees and grape species differ as to their susceptibility to Fe chlorosis, but it is widely accepted that peach, pear, and and kiwifruit are among the most susceptible to Fe chlorosis (Korcak, 1987). Vitis spp. also differ as to their susceptibility, some being very tolerant (e.g. V. _inifera and V. rupestris) others being susceptible (e.g. V. riparia).
abstract
Several perennial, deciduous, as well as evergreen fruit crops develop symptoms of iron deficiency— interveinal chlorosis of apical leaves— when cultivated in calcareous and alkaline soils. Under these conditions fruit yield and quality is depressed in the current year and fruit buds poorly develop for following year fruiting. This paper reviews the main fundamental and applied aspects of iron (Fe) nutrition of deciduous fruit crops and grapevine and discusses the possible development of sustainable Fe nutrition management in orchard and vineyard ecosystems. Cultivated grapevines and most deciduous fruit trees are made up of two separate genotypes the cultivar and the rootstock, providing the root system to the tree. The effect of the rootstock on scion tolerance of Fe chlorosis is discussed in terms of biochemical responses of the roots to acquire iron from the soil. Symptoms of iron chlorosis in orchards and vineyards are usually more frequent in spring when shoot growth is rapid and bicarbonate concentration in the soil solution buffers soil pH in the rhizosphere and root apoplast. Since the solubility of Fe-oxides is pH dependent, under alkaline and calcareous soils inorganic Fe availability is far below that required to satisfy plant demand, so major role on Fe nutrition of trees is likely played by the iron chelated by microbial siderophores, chelated by phytosiderophores (released into the soil by graminaceous species) and complexed by organic matter. As most fruit tree species belong to Strategy I-based plants (which do not produce phytosiderophores in their roots) Fe uptake is preceded by a reduction step from Fe3+ to Fe2+. The role of ferric chelate reductase and proton pump activities in Fe uptake and the possible adoption of these measurements for screening procedure in selecting Fe chlorosis tolerant rootstocks are discussed. In a chlorotic leaf the existence of Fe pools which are somehow inactivated has been demonstrated, suggesting that part of the Fe coming from the roots does not pass the leaf plasmamembrane and may be confined to the apoplast; the reasons and the importance for inactivation of Fe in the apoplast are discussed. The use of Fe chlorosis tolerant genotypes as rootstocks in orchards and vineyards represents a reliable solution to prevent iron chlorosis; in some species, however, available Fe chlorosis resistant rootstocks are not very attractive from an agronomic point of view since they often induce excessive growth of the scion and reduce fruit yields. As most fruit tree crops and grapes are high value commodities, in many countries growers are often willing to apply synthetic Fe chelates to cure or to prevent the occurrence of Fe deficiency. The application of iron chelates does not represent a
…
Iron chlorosis is a more complex phenomenon in fruit trees than in annual crops (Tagliavini et al., 2000a). Deciduous fruit trees and grapes ex- hibit reproductive cycles starting with bud forma- tion in one year and ending with flowering, fruit set and fruit maturity the following year. Esti- mates of chlorosis severity among pear orchards in the Po Valley (Northern Italy) (Scudellari, 1999, personal communication) suggest that trees bearing a large amount of fruits in one year are more prone to show a severe chlorosis development the following year. This phenomenon, in accordance with findings by Pouget (1974), has been explained considering the fact that fruits represent a strong sink for carbohydrates, of which storage at root level might be insufficient to sustain root growth and activity during growth resumption in spring. In this context it is of interest that Fe is only taken up by root tips (Clarkson and Hanson, 1980) and, therefore, the number of root tips produced by a rootstock in spring may have an influence on Fe uptake.
Another peculiar aspect of Fe nutrition in trees is related to their size and to the fact that, after absorption, Fe has to be transported for a long distance to reach the tree canopy. Problems in Fe transport through the xylem are, therefore, more likely in mature trees. Symptoms of iron chloro- sis-typical interveinal yellowing or sometimes atypical uniform chlorosis as in pear-in orchards and vineyards often start as soon as buds open, likely as a result of insufficient storage of Fe, or develop throughout the vegetative season as a consequence of plant demand being excessive in respect to Fe availability. In general, however, chlorosis occurs more frequently in spring when rainfalls cause a raise in soil bicarbonate concen- tration (Boxma, 1982) in a period of intense Fe demand. If soil conditions then improve, new leaves appear green, but those previously chlorotic unlikely re-green. Fruit yield losses caused by leaf chlorosis also depend on the degree and the period the chlorosis develop and, in gen- eral, critical periods coincide with blooming and fruit set: this particularly applies to fruits not easy to set, like pears (Pyrus communis) or those like kiwifruit (Actinidia deliciosa), of which the final size strongly depends on seed number. A rela- tively low level of chlorosis is likely more accept- able at other phenological phases, especially if it is confined to parts of the canopy where only vege- tative and not reproductive buds are present.
Chlorotic symptoms also vary from year to year as a result of several tree and environmental variables, like yields, temperatures, rains. In soils where shallow layers are less rich in CaCO3 than deeper layers, it is likely that trees and vines develops chlorosis only when they age and roots explore layers with poor conditions for Fe uptake.
Studying the characteristics of soil profile pro- vides a useful tool for understanding such prob- lems. To our experience, soils which had been for many years subjected to ploughing before the plantation may present layers of fine texture, just below the ploughing depth, which could be rich in CaCO3 because of leaching from more shallow layers.
Symptoms of iron chlorosis are not always uniform within a single tree, where chlorotic parts of the canopy are often present together with green branches. Little attention has been given to explaining this phenomenon, but few hypotheses can be made: (1) tree root systems exploit a relatively large volume of soil with heterogeneous characteristics, some roots developing in mi- crosites favourable to Fe uptake while others are located in poor areas. Due to the poor phloem mobility of the iron absorbed by one root, redis- tribution of iron from green canopy parts to chlorotic ones is unlikely; (2) since iron is mainly transported in a non-ionic form in the xylem (Tiffin, 1970) its transport, driven by the transpi- ration stream, is likely not uniform. It has been reported (Tagliavini et al., 2000a) that within the same tree or vine, leaf Fe chlorosis is often more severe on second or third order branches than on those directly branching off the trunk.
Problems in transport of Fe may also arise from the fact that in some species (e.g. pear), a certain degree of scion/rootstock (usually quince) incompatibility occurs and is sometimes desirable as it allows a control of the tree size. Graft incompatibility, however, impairs both upward nutrient transportation through the xylem and downward carbohydrates replenishment to the roots through the phloem (Breen, 1975). Under these conditions, root reactions to Fe deficiency, such as the increase of Fe-reduction activity and proton extrusion (Mengel and Malissovas, 1982), may be impaired because of a lack of organic carbon supplied from the shoots.
Iron demands by mature orchards and vine- yards are in the range of 650 – 1100 g Fe ha−1 per year (Ga¨rtel, 1993): net removals are mainly ac- counted by amounts recovered in yields and prun- ing wood, if not left in the ground and chopped, while the Fe amounts in the perennial framework,
with a relatively constant biomass do not signifi- cantly vary from year to year and are, therefore, negligible. For kiwifruit, fruit Fe concentrations of 33 µg g−1 (DW) were estimated, resulting in a total removal of Fe in fruits of around 160 g ha−1 for a fruit production of 30 t ha−1. For several flesh fruit crops (Tagliavini et al., 2000b) removals are in the range of 1 – 10 g t−1 of harvested yield. Annual removal of Fe by pruning wood were estimated by Abadia et al. (personal communica- tion, 2000) for peach trees in Northern Spain being in the order of 150 g Fe ha−1 and similar Fe amounts were estimated returning to the soil through the leaves after their abscission.
2.2. Soil iron availability
As previously described, annual removals of iron in orchards and vineyards are relatively low. Total amounts of iron in cultivated soils, in the- ory would not justify the development of iron deficiency, which, nevertheless, often occurs as a result of poor availability of iron for plants. Sev- eral soil-related characteristics may lead to devel- opment of iron chlorosis (Table 1).
The prediction of risks of future development of iron chlorosis in a plantation is of great impor- tance in fruit tree and grape industry and should lead to the correct choice of the rootstock to be used. Mistakes at this stage would make unlikely the achievement of satisfactory yields without agronomic and chemical means for correcting the chlorosis throughout the life span of the orchard or vineyard. Due to the number of soil factors that impair Fe nutrition, it is not always easy to predict the possible chlorosis development of a perennial crop on the basis of a single soil parameter. Soil pH is often a useful but not sufficient parameter: it is well known that fruit crops adapted to acidic soils quickly develop chlorosis at sub-alkaline or alkaline conditions (e.g. blueberry, raspberry, kiwifruit) while other genotypes are more able to cope with high soil pH (likely through an inherent ability to lower root apoplastic pH), unless the soil is also calcareous and, therefore, buffered in the range of 7.5 – 8.5 (Loeppert et al., 1994). Total lime, however, is not particularly useful for predicting the development of iron chlorosis, while the fine, clay-sized, frac- tion of CaCO3, active carbonate or active lime (Drouineau, 1942), is more reactive and, there \ fore, able to build and maintain high levels of HCO− in the soil solution (Inskeep and Bloom, 1986), and is, therefore, often a more reliable (than High soil pH) indicator. Species are ranked according to the level of active lime at which they start to develop chlorotic symptoms: very susceptible species or
…
Other iron containing compounds
Ferrous sulphate, either applied to the soil or to the tree (leaf treatments, trunk injection), has been the major therapy against Fe chlorosis from the first description of this nutritional disorder until the introduction of Fe synthetic chelates and is still widely used by fruit growers especially in the developing countries due to its low costs. If supplied alone, however, soil applied Fe(II)sulphate is of little or no agronomic value in calcareous soils where the Fe2+ is subject to rapid oxidation and insolubilisation as hydroxide. For example, Fe sulphate was not effective for curing Fe chlorosis in A. deliciosa in a soil with a high CaCO3 content (32%), while a quite complete recovery was achieved by Fe– EDDHA (Loupas- saki et al., 1997).
The effectiveness of soil applied Fe sulfate may be improved by combining iron sulphate with organic substrates able to complex the Fe (e.g. animal manures, sewage sludges, compost, peat, plant residues). Canopy applied Fe sulphate also represents a valuable, inexpensive, alternative to foliar applied synthetic Fe chelates (Tagliavini et al., 2000a) to cure iron chlorosis.
soil injection of vivianite was slightly less effec- tive, but showed a more lasting impact. Vivianite application has the advantage of being relatively inexpensive and is directly prepared by growers simply mixing ferrous sulphate with di-ammo- nium (or mono-ammonium) phosphate (Rosado et al., 2000). The effectiveness of soil applied Fe amorphous minerals is presumably due to the fact that they are more easily mobilised by plants and microorganisms as compared with crystalline Fe forms (Loeppert et al., 1994).
Use of vivianite (Fe3(PO4)2.8H2O) to prevent iron chlorosis in calcareous soils
For various reasons, iron phosphate might be effective in correcting Fe chlorosis in calcareous soils. To test this hypothesis, several pot experiments were conducted using an Fe chlorosis-sensitive chickpea (Cicer arietinum L.) cultivar cropped in soils to which partially oxidized vivianites (Fe3(PO4)2.8H2O) and Fe(III) phosphates with different characteristics had been added. Vivianites mixed with the soil at a rate of 1 g kg−1 were as effective in preventing chlorosis as Fe chelate (FeEDDHA). However, the effectiveness of Fe(III) phosphates was less, suggesting that the presence of Fe(II) in the phosphates used was a key factor in their Fe-supplying value to plants. The effectiveness of vivianites, however, seemed to be largely independent of their Fe(II) content.
Citrus Fertilizer Management on Calcareous Soils (pdf)
Citrus fertilizer management on calcareous soils differs from that on noncalcareous soils because of the effect of soil pH on soil nutrient availability and chemical reactions that affect the loss or fixation of some nutrients. The presence of CaCO3 directly or indirectly affects the chemistry and availability of nitrogen (N), phosphorus (P), magnesium (Mg), potassium (K), manganese (Mn), zinc (Zn), and iron (Fe). The availability of soil copper (Cu) is also affected; however, since the citrus Cu requirement is normally satisfied through foliar sprays of Cu fungicides, it is not discussed in this fact sheet. concentration, unless a significant quantity of sodium (Na) is present.
Many Florida flatwoods soils contain one or more calcareous horizons, or layers (see Table 1). A typical characteristic is an alkaline, loamy horizon less than 40 inches deep, which can be brought to the surface during land prepara- tion for citrus planting. These soils are important for citrus production in the Indian River area (east coast) and, to
Soil pH affects the rates of several reactions involving N and can influence the efficiency of N use by plants. Nitrifica- tion, or the conversion of ammonium (NH +) to nitrate (NO -) by soil bacteria, is most rapid in soils with pH values between 7 and 8. Nitrification approaches zero below pH
Ammonium-N fertilizers applied to calcareous soils are converted within a few days to nitrate, which moves freely with soil water. The acidity produced during nitrification is quickly neutralized in highly calcareous soils but may lower the pH value in soils containing low levels of CaCO3.
Ammonia volatilization is the loss of N to the atmosphere through conversion of the ammonium ion to ammonia gas (NH3). Volatilization of ammoniacal-N fertilizer is signifi- cant only if the soil surface pH value is greater than 7 where the fertilizer is applied. This condition occurs in calcareous soils, or where the breakdown of the N fertilizer produces alkaline conditions (e.g., urea decomposition). Nitrogen loss through ammonia volatilization on calcareous soils is
a concern when ammoniacal N is applied to the grove floor and remains there without moving into the soil. Following an application of dry fertilizer containing ammoniacal N, the fertilizer should be moved into the root zone through irrigation or mechanical incorporation if rainfall is not imminent. Since urea breakdown creates alkaline condi- tions near the fertilizer particle, surface application of urea can cause N loss if the urea is not incorporated or irrigated into the soil, regardless of initial soil pH.
The Effect of CaCO3 On Magnesium and Potassium
Although low concentrations of Mg and K in citrus leaves are not uncommon in groves planted on calcareous soils, there is not necessarily a concurrent reduction in fruit yield or quality. If a low concentration of leaf K or Mg is found in a grove that produces satisfactory yield and quality, attempts to increase leaf levels with fertilizer are not neces- sary. However, if a detrimental condition such as low yield, small fruit, or creasing is observed, an attempt to raise the leaf K or Mg concentration with fertilizer is justified.
It is often difficult to increase leaf Mg and K levels with fertilizer applied directly to calcareous soils, which contain tremendous quantities of both exchangeable and nonexchangeable Ca. Leaf Mg and K concentrations are strongly influenced by soil conditions that control leaf Ca
concentration, including high soil Ca levels. High Ca levels suppress Mg and K uptake by citrus trees in part, presum- ably, through the competition of Ca2+, Mg2+, and K+. Citrus growing on soils high in Ca often requires above normal levels of Mg and K fertilizer for satisfactory tree nutrition. In cases where soil-applied fertilizer is ineffective, the only means of increasing leaf Mg or K concentration may be through foliar application of water-soluble fertilizers, such as magnesium nitrate [Mg(NO3)2] or potassium nitrate (KNO3).
The Effect of CaCO3 On Phosphorus
Phosphorus availability in calcareous soils is almost always limited. The P concentration in the soil solution is the factor most closely related to P availability to plants. The sustainable concentration is related to the solid forms of
P that dissolve to replenish soil solution P following its depletion by crop uptake. Many different solid forms of phosphorus exist in combination with Ca in calcareous soils. After P fertilizer is added to a calcareous soil, it undergoes a series of chemical reactions with Ca that decrease its solubility with time (a process referred to as P fixation). Consequently, the long-term availability of P to plants is controlled by the application rate of soluble P and the dissolution of fixed P. Applied P is available to replenish the soil solution for only a relatively short time before it converts to less soluble forms of P.
The Effect of CaCO3 On Zinc and Manganese
Soil pH is the most important factor regulating Zn and Mn supply in alkaline soils. At alkaline (high) pH values, Zn and Mn form precipitous compounds with low water solubility, markedly decreasing their availability to plants. A soil pH value of less than 7 is preferred to ensure that Zn
chlorosis can be corrected by using organic chelates, a method discussed in detail in a later section.
…
Summary
- Calcareous soils are alkaline because they contain CaCO3. They are commonly found in south Florida citrus groves, especially in the Indian River area.
- The availability of N, P, K, Mg, Mn, Zn, and Fe to citrus decreases when soil CaCO3 concentration increases to more than about 3% by weight. These soils generally have a pH value in the range of 7.6 to 8.3.
- To avoid ammonia volatilization, fertilizers containing ammonium-N or urea should be moved into the root
- where soil applications are not.
- The least expensive and more efficient way to correct Zn and Mn deficiencies of citrus in calcareous soils is through foliar application of inorganic or organically chelated forms.
- The easiest way to avoid lime-induced Fe chlorosis on calcareous soils is to plant trees budded on tolerant rootstocks.
- The most effective remedy for lime-induced Fe chlorosis on nontolerant rootstocks involves the use of organically chelated Fe.
- Sulfur products that act as soil acidulents can potentially improve nutrient availability in calcareous soils.
============================================
The Real Dirt on Austin Area Soils
The Austin area is home to three ecoregions that have very different types of soil; the Edwards Plateau, the Blackland Prairies, and the Post Oak Savannah Floodplains. All of them are somewhat alkaline, have challenging clay issues, and are low in organic matter. We’ll describe each region to help you identify which one you are gardening in and give you some tips for success. Here is a link to Soil Survey of Travis County Texas if you want to deep dive even further to see the variability in the Austin area soils.

Edwards Plateau Description and Gardening Strategies

The Edwards Plateau is characterized by thin soils on top of exposed limestone
I-35 helps to physically separate the western Edwards Plateau from the eastern Blackland Prairies region here in Austin. This is the reason that garden center employees and the Travis County Master Gardeners ask you which side of the freeway you live on!
You know you’re on the Edwards Plateau if you can observe the following:
- Exposed Limestone
- Soil contains large amounts of crumbled limestone, referred to as calcareous rubble
- Clay soil prevalent
The biggest challenge is not having enough soil. Sometimes builders will bring in soil for new housing developments, but it’s usually not very much and sometimes can be from poor materials like sandy clay. Your best bet is to prioritize what you want to grow and think of your landscape as planting islands. Planting islands allow you to concentrate on soil improvement or find affordable ways to purchase more soil.
Slow Down Runoff
The thin soils and elevation changes contained on the Edwards Plateau make this area prone to runoff. Evaluate your gardening site for ways to slow down the runoff. You can do this with physical barriers, tiers, raised beds, and retaining areas like rain gardens. Utilize materials like larger stones and coarsely ground mulch for transition zones to help prevent decomposed granite or soil mixes from washing away. Summer and Winter cover crops also help stabilize things and keep bare soil in place. An added benefit is that roots help to break down limestone. The clumping and fibrous nature of native prairie grass roots are also good plant choices to help break down limestone.
Add Organic Matter for Soil Health
All clay soils are deficient in organic matter, and especially so on the Edwards Plateau. But the trick is not to go overboard. Your garden soil should contain at least 30% by volume of real soil. This mineralized content is vital for plant health. If you need to purchase soil, make sure that it is not a soilless mixture like a potting mix. Look for words like “top soil” and ask to see the ingredients listing.
Improve the soil that you have with one to two inches of compost added to the topsoil each spring and fall. You’ll also want to retain soil moisture by using three inches of mulch. These thin soil layers leave plants susceptible to summer drought when the root zone can quickly deplete the soils limited moisture reserves. The mulch will eventually break down and become compost, so you may need to add more each planting season. When applying mulch, be sure to give your plants some space by allowing a gap between the mulch and the main stems of the plant.